Clinical Resources
Materials for Providers
Alphabetical by Title
Standing orders for Administering Measles, Mumps, and Rubella Vaccine to Adults
Eligible healthcare professionals may vaccinate adults who meet any of the criteria on this form
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
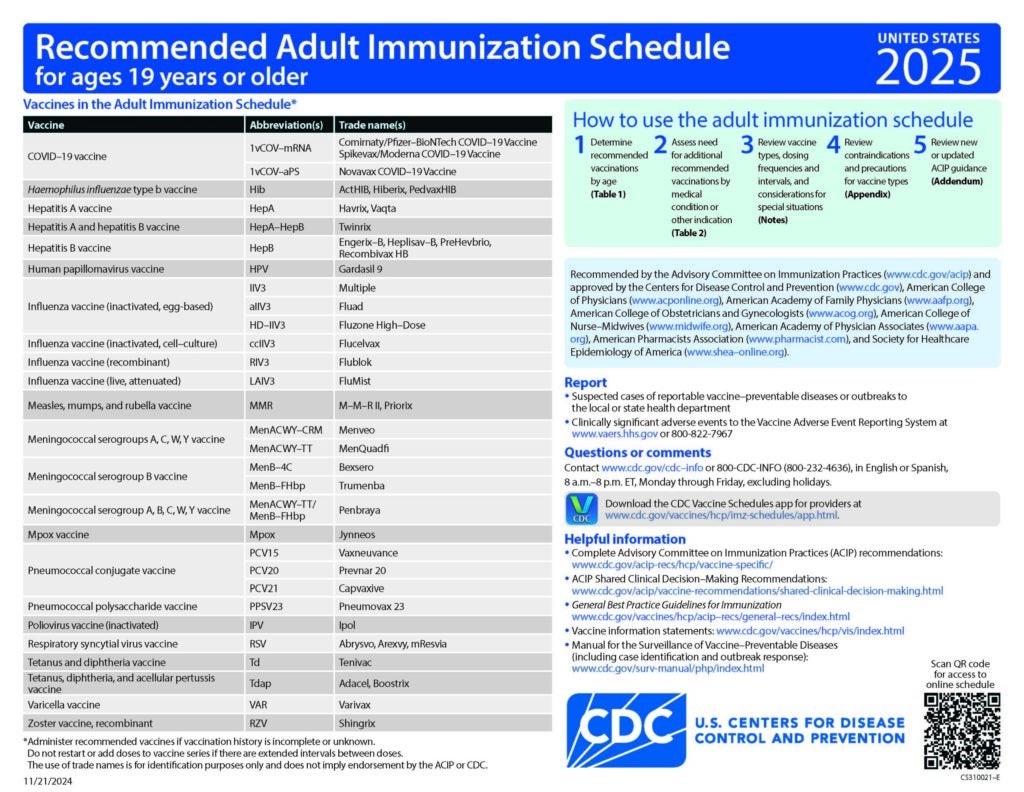
CDC Recommended Schedules
FDA Package Inserts & EUAs
State Policies
Healthcare Professional Organizations
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Travelers’ health information for healthcare providers
Measles prevention guidance for international travelers