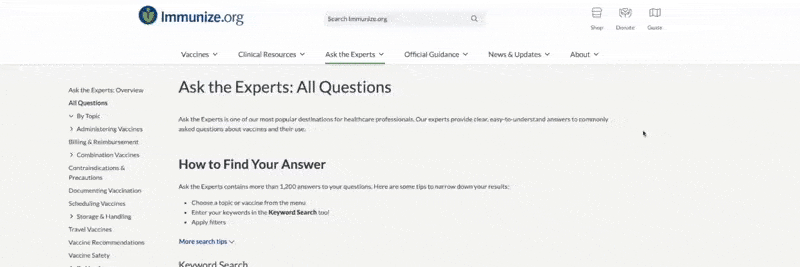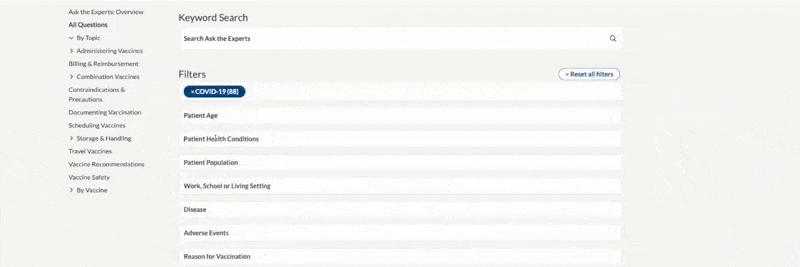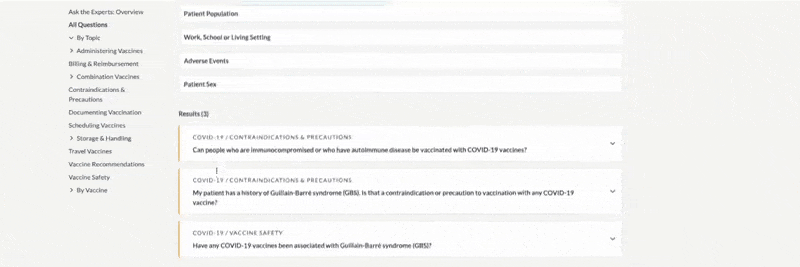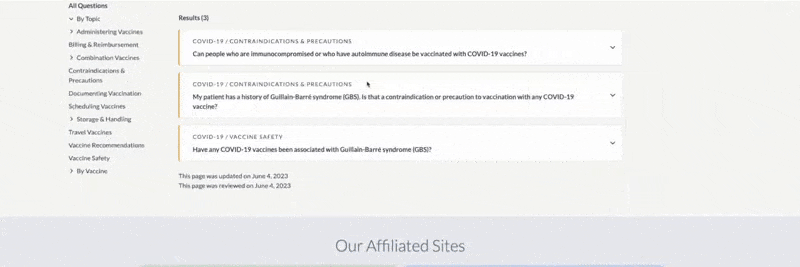
FDA Approval –
August 27, 2025
FDA MedWatch –
August 22, 2025
FDA –
August 6, 2025
FDA Approval –
July 24, 2025
FDA Approval –
July 9, 2025
FDA Safety Communication –
June 25, 2025
FDA Approval –
June 12, 2025
FDA Approval –
June 9, 2025
FDA Approval –
May 30, 2025
FDA Approval –
May 23, 2025
FDA –
May 22, 2025
FDA Approval –
May 16, 2025
FDA MedWatch –
May 12, 2025
FDA –
May 9, 2025
FDA Approval –
March 31, 2025
FDA –
March 13, 2025
FDA Approval –
February 14, 2025
FDA Approval –
February 14, 2025
FDA News Release –
January 7, 2025
FDA –
November 29, 2024
FDA Approval –
October 22, 2024
FDA Approval –
October 21, 2024
FDA Approval –
October 18, 2024
FDA –
September 20, 2024
FDA –
September 18, 2024
FDA –
September 11, 2024
FDA Approval –
August 30, 2024
FDA Approval –
August 29, 2024
FDA Approval –
August 22, 2024
FDA Approval –
August 19, 2024
FDA Approval –
June 17, 2024
FDA Approval –
June 13, 2024
FDA Approval –
June 10, 2024
FDA Approval –
May 31, 2024
FDA Approval –
November 9, 2023
FDA News Release –
November 1, 2023
FDA Approval –
October 20, 2023
FDA –
October 3, 2023
FDA Approval –
September 11, 2023
FDA Approval –
August 21, 2023
FDA Approval –
July 27, 2023
FDA Approval –
July 20, 2023
FDA Approval –
July 17, 2023
FDA Approval –
May 31, 2023
FDA Approval –
May 3, 2023
FDA Approval –
April 28, 2023
FDA Approval –
April 27, 2023
FDA Approval –
April 18, 2023
FDA Approval –
March 14, 2023
FDA Approval –
December 8, 2022
FDA Approval –
October 19, 2022
FDA Approval –
October 14, 2022
FDA Approval –
October 12, 2022
FDA Approval –
October 7, 2022
FDA Approval –
August 31, 2022
FDA Approval –
August 19, 2022
FDA Approval –
August 9, 2022
FDA Approval –
July 13, 2022
FDA Approval –
July 8, 2022
FDA Approval –
June 17, 2022
FDA Approval –
June 17, 2022
FDA Approval –
June 17, 2022
FDA Approval –
June 3, 2022
FDA Approval –
May 17, 2022
FDA Approval –
May 5, 2022