Clinical Resources
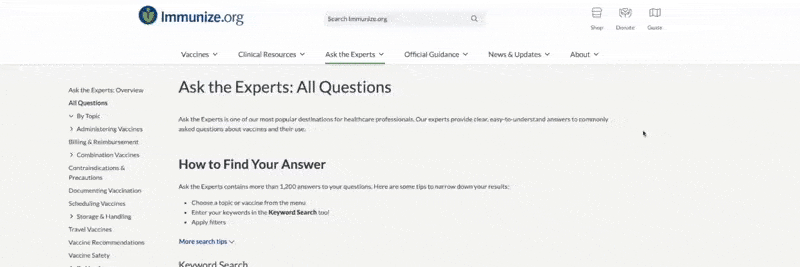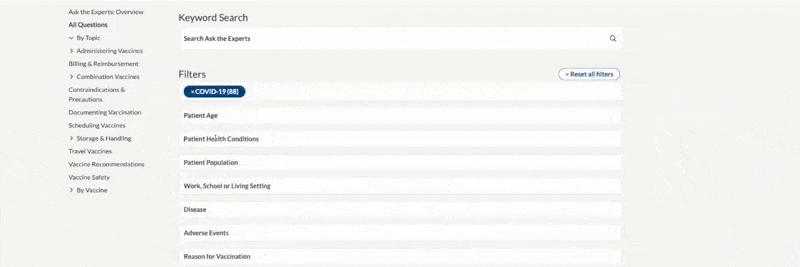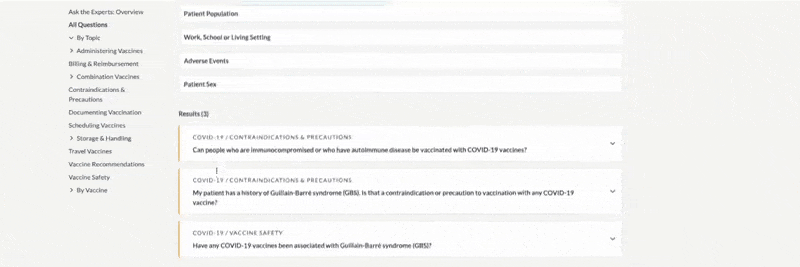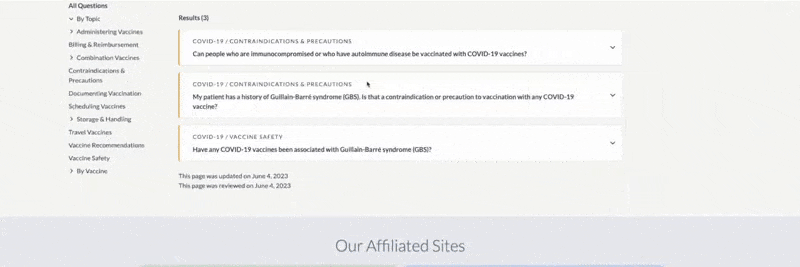
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
FDA Package Inserts & EUAs
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Travelers’ health information for healthcare providers
Rabies status information by country