Clinical Resources
Materials for Providers
Alphabetical by Title
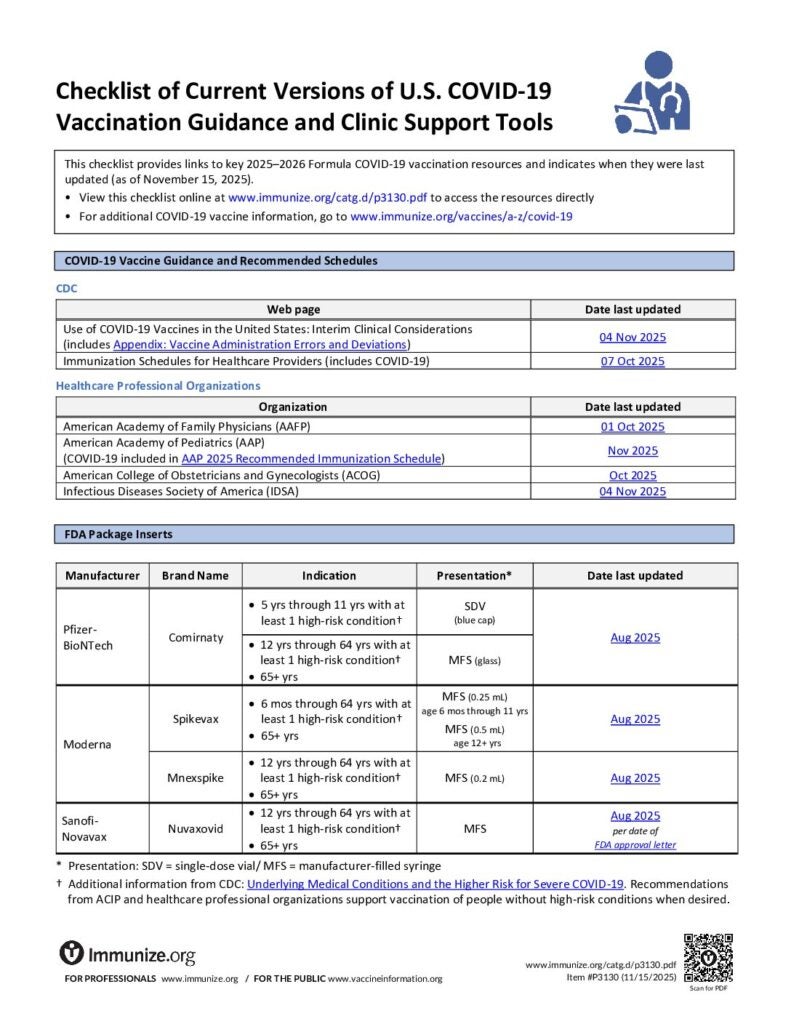
Checklist of Current Versions of U.S. COVID-19 Vaccination Guidance and Clinic Support Tools
Checklist of links to key COVID-19 vaccination resources, including the date they were last revised. Updated at least monthly.
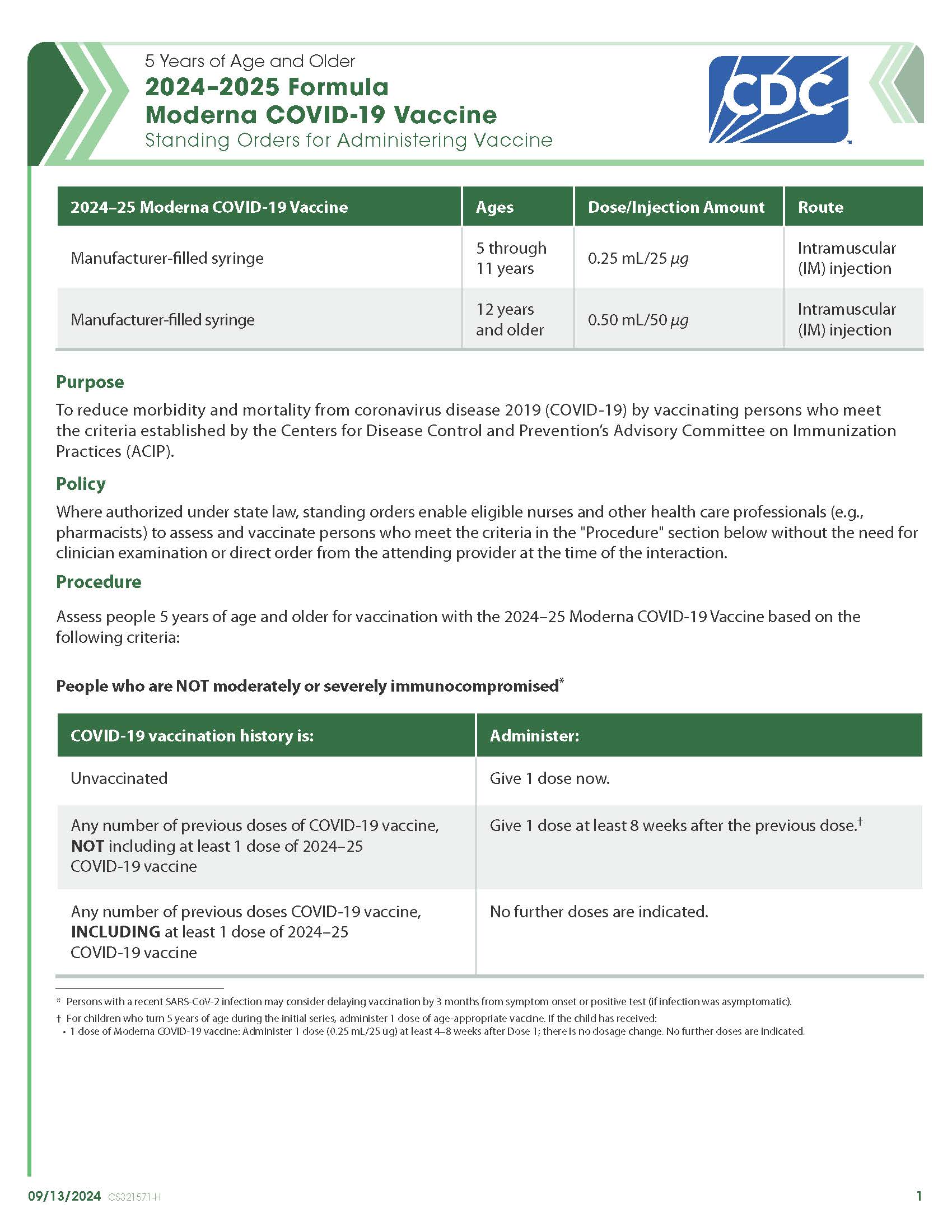
COVID-19 Moderna (2024–2025 Formula) Vaccine — 5 Years of Age and Older (Age 5 Through 11 Yrs – Manufacturer-Filled Syringe; Age 12+ Yrs Manufacturer-Filled Syringe)
CDC’s form: “5 Years of Age and Older 2024–2025 Formula Moderna COVID-19 Vaccine Standing Orders for Administering Vaccine”
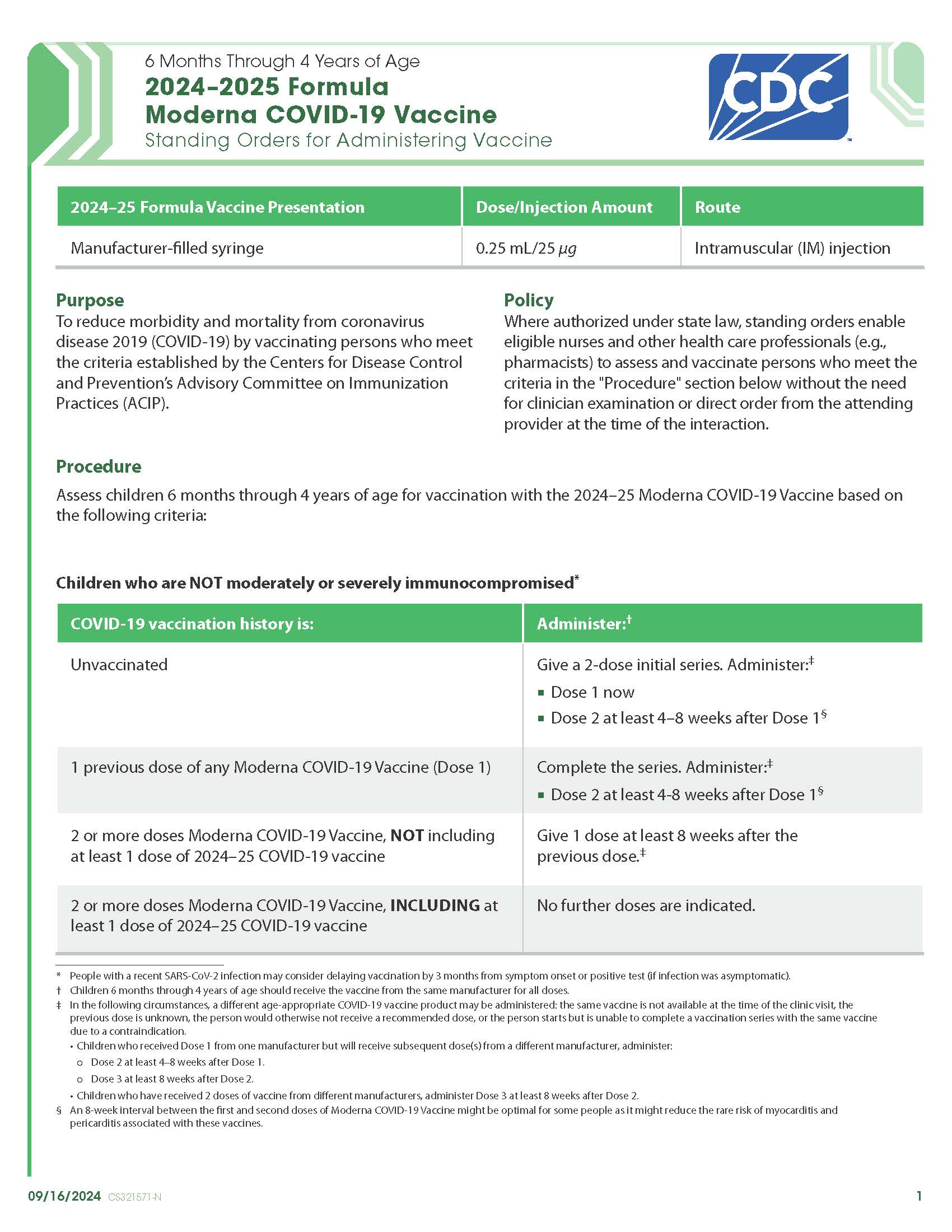
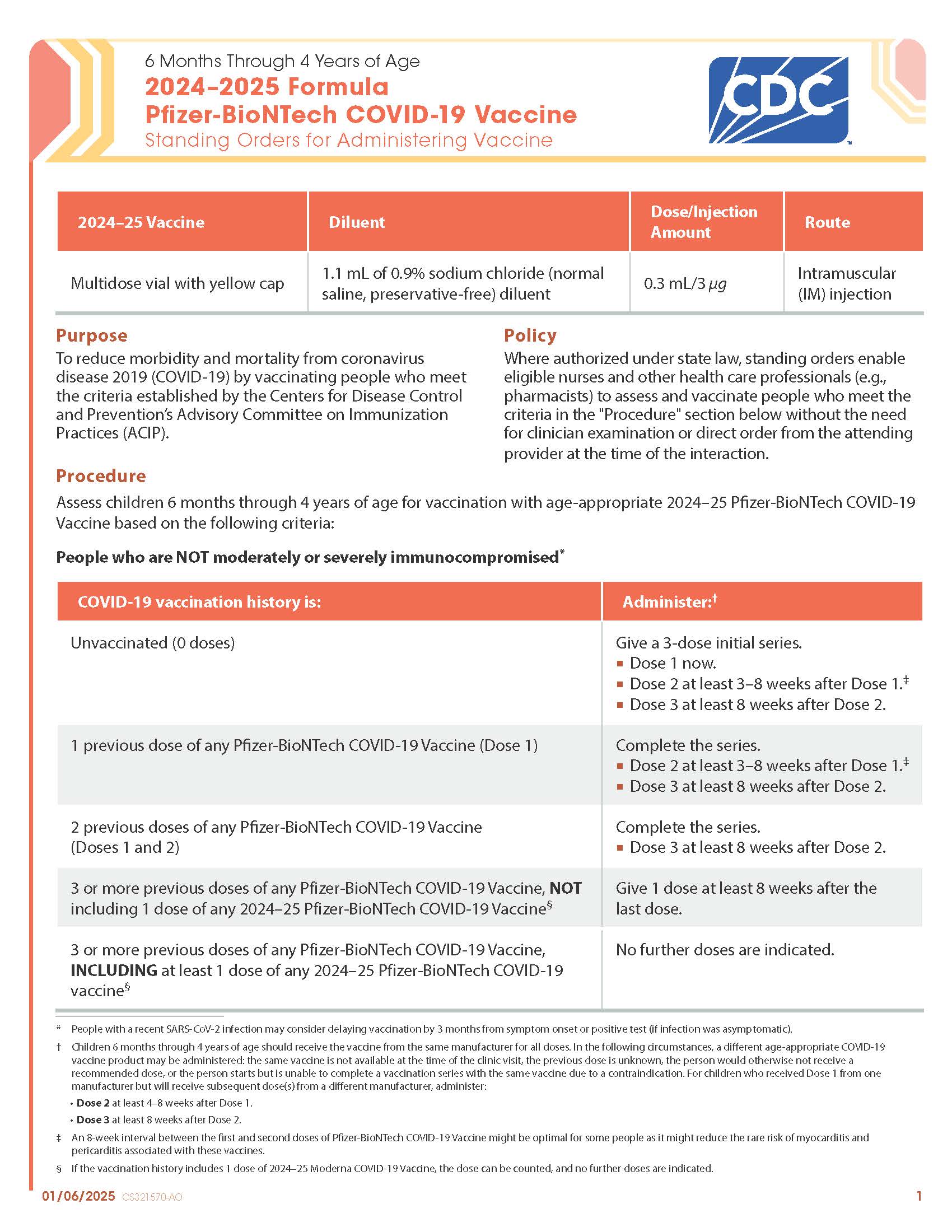
COVID-19 Moderna (2024–2025 Formula) Vaccine — 6 Months Through 4 Years of Age (Manufacturer-Filled Syringe)
CDC’s form: “6 Months Through 4 Years of Age 2024–2025 Formula Moderna COVID-19 Vaccine Standing Orders for Administering Vaccine”
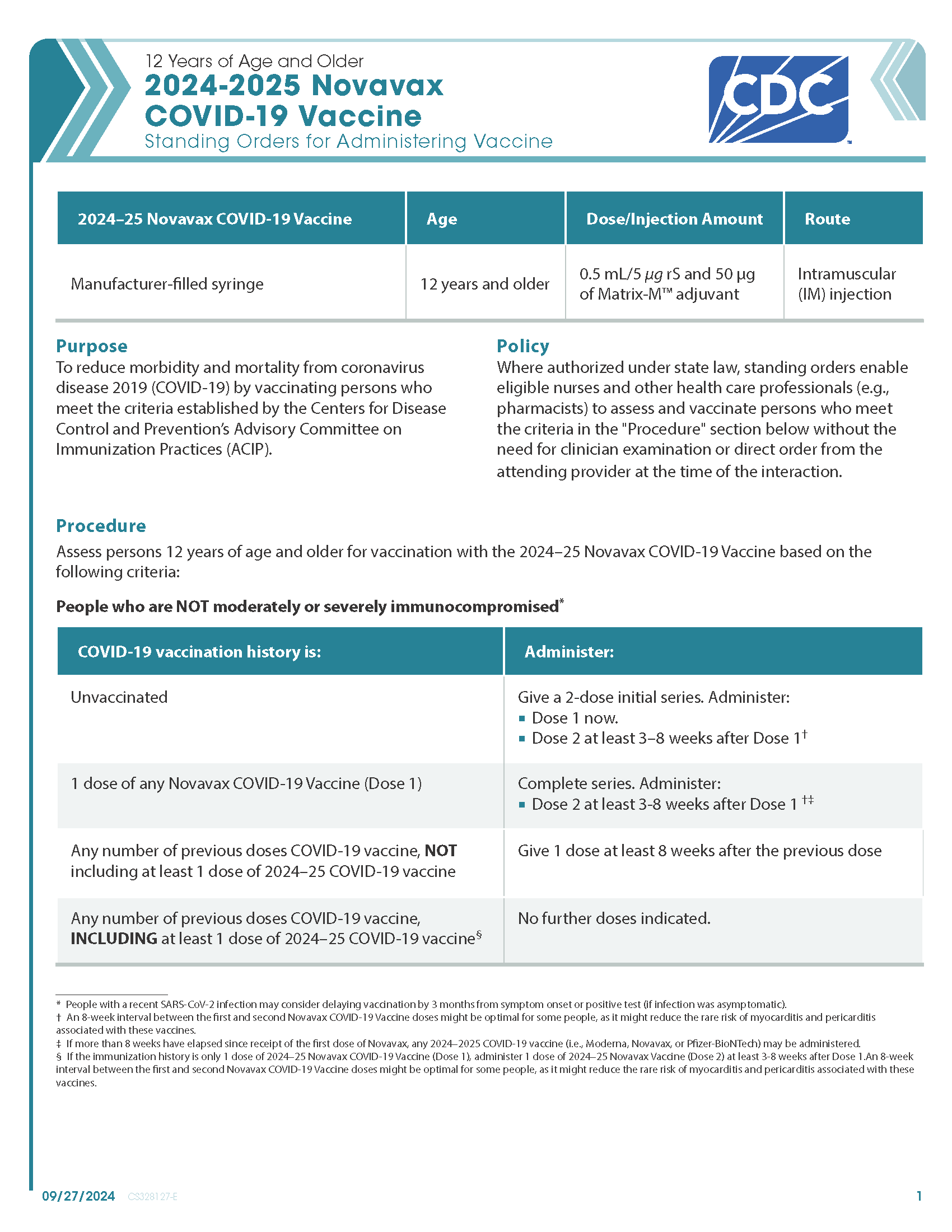
COVID-19 Novavax (2024–2025 Formula) Vaccine — 12 Years of Age and Older (Manufacturer-Filled Syringe)
CDC’s form: “12 Years of Age and Older 2024–2025 Novavax COVID-19 Vaccine Standing Orders for Administering Vaccine”
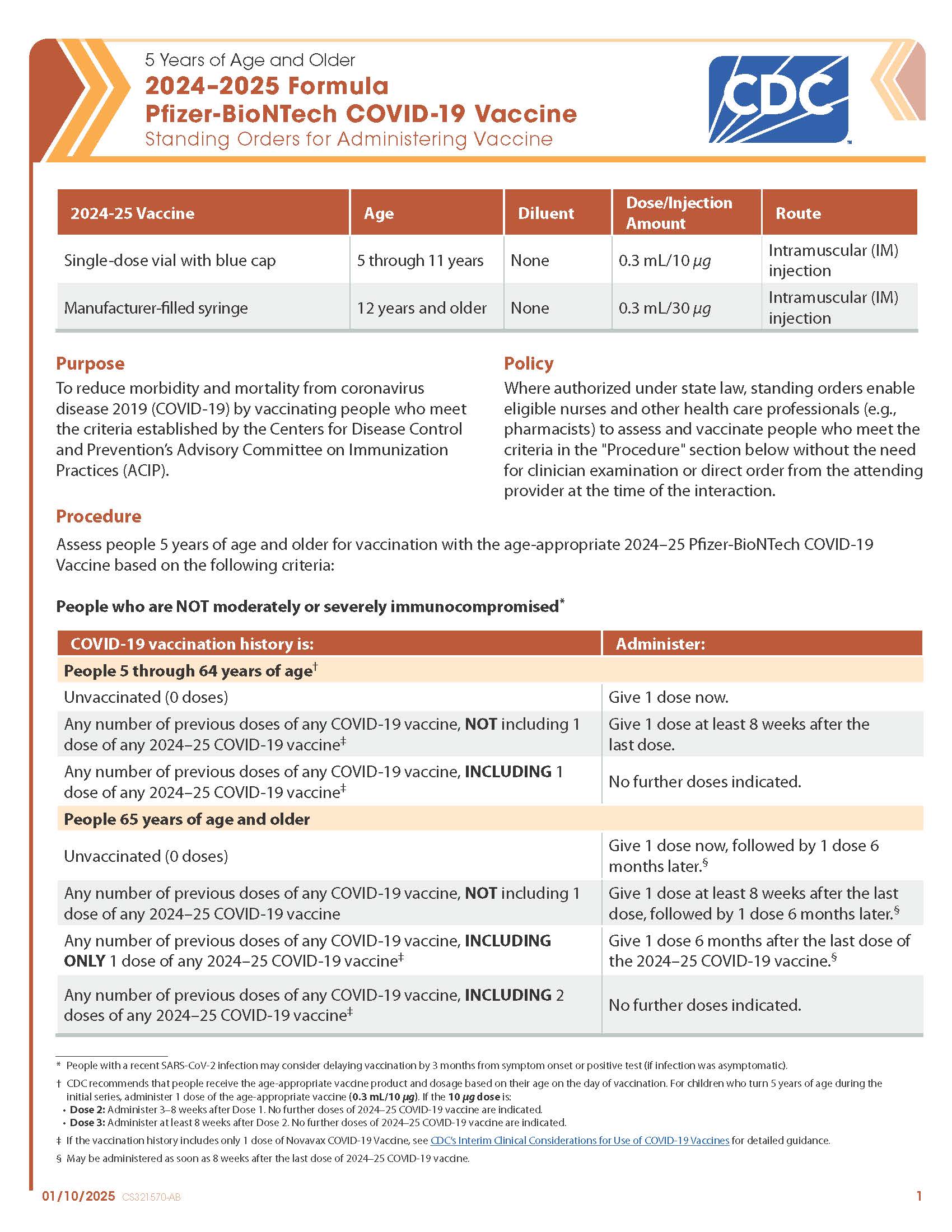
COVID-19 Pfizer-BioNTech (2024–2025 Formula) Vaccine — 5 Years of Age and Older (Blue Cap Single Dose Vial, Manufacturer-filled Syringe)
CDC’s form: “5 Years of Age and Older 2024–2025 Formula Pfizer-BioNTech COVID-19 Vaccine Standing Orders for Administering Vaccine”
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Additional Federal Resources
- All current and archived ACIP COVID-19 recommendations
- ACIP COVID-19 recommendations at CDC
- Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States (CDC)
- COVID-19 Vaccination Clinical & Professional Resources (CDC) [main page]
- U.S. COVID-19 Vaccine Product Information (CDC)
- Clinical Care Considerations for COVID-19 Vaccination (CDC)
- Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines Among Adolescents and Young Adults (CDC)
- Vaccinate with Confidence: Strategy to Reinforce Confidence in COVID-19 Vaccines (CDC)
- Ensuring COVID-19 Vaccine Safety in the US (CDC)
- Vaccine Storage and Handling Toolkit (CDC): COVID-19 vaccine information found in addendum
- Multilingual COVID-19 Resources (FDA)
- General Best Practice Guidelines for Immunization
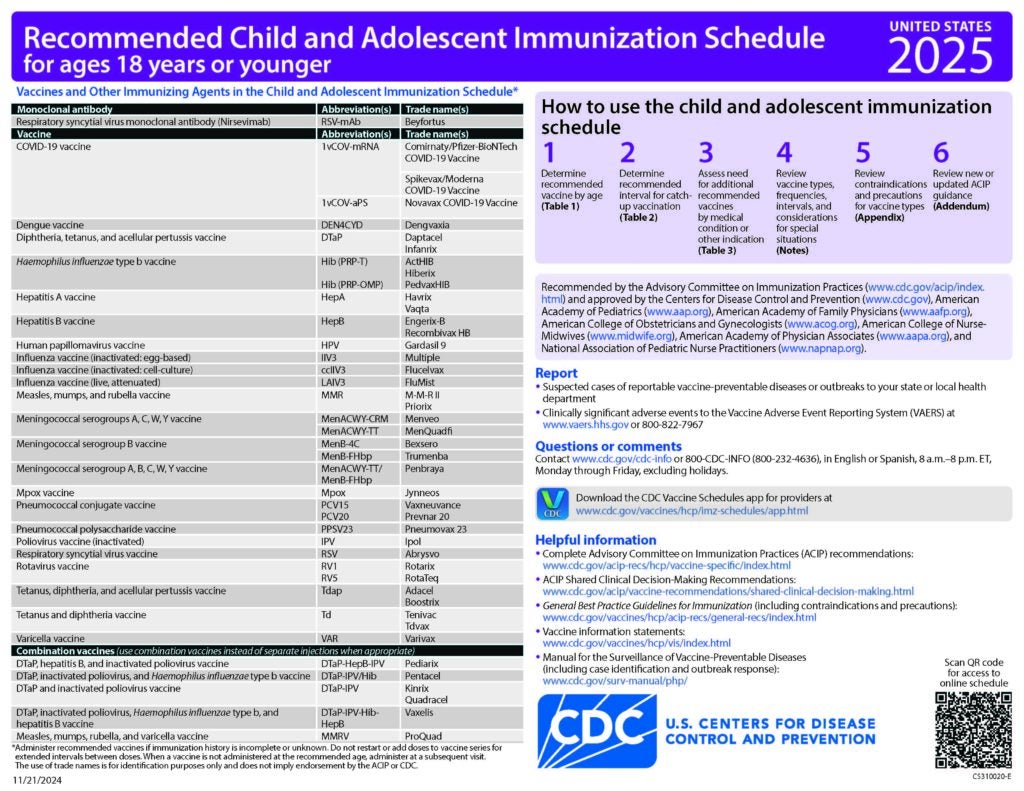
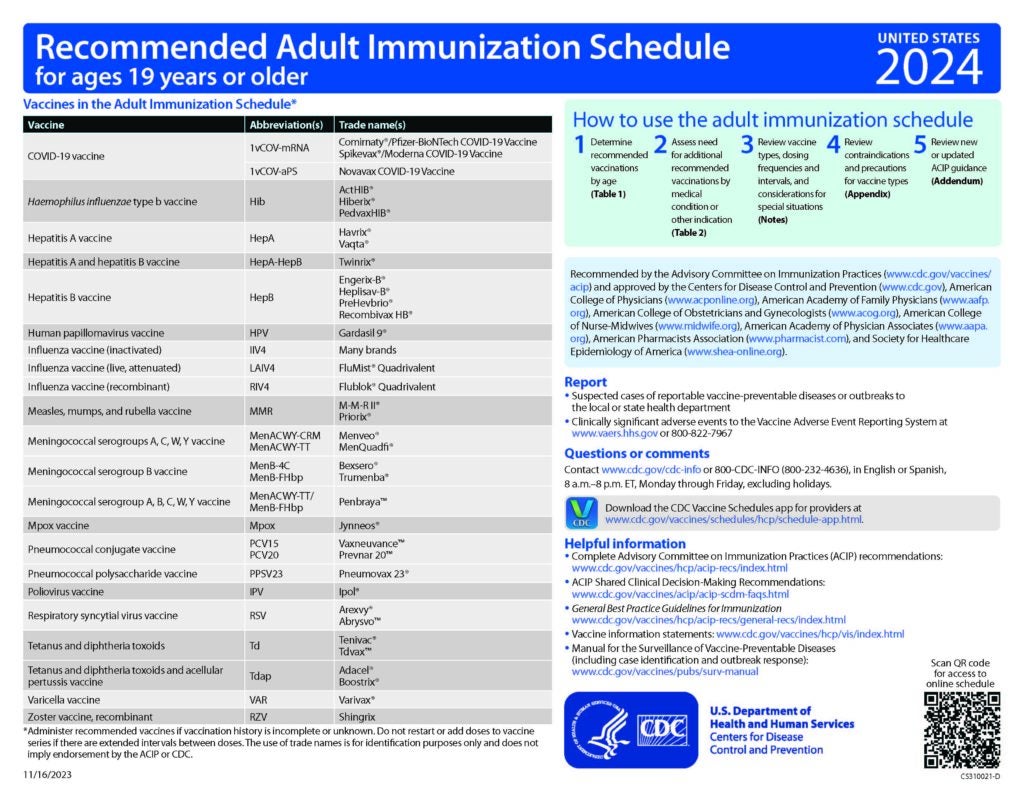
CDC Recommended Schedules
FDA Package Inserts & Vaccine Fact Sheets (EUA)
State Policies
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Travelers’ health information for healthcare providers