Clinical Resources
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
ACIP Recommendations for Use of Recombinant Zoster Vaccine (RZV, Shingrix, GSK) in Immunocompromised Adults aged 19 years and older, US, 2022
MMWR, January 21, 2022, 71(3);80-84
Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines
MMWR, January 26, 2018; 67(3); 103–108
Prevention of Herpes Zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, June 6, 2008; 57(05):1-30
Additional Federal Resources
- All current and archived ACIP Zoster (Shingles) recommendations
- General Best Practice Guidelines for Immunization
- ACIP Zoster (Shingles) recommendations at CDC
- CDC Zoster (Shingles) Information for Healthcare Professionals
- Clinical Considerations for Use of Recombinant Zoster Vaccine (RZV, Shingrix) in Immunocompromised Adults ≥19 Years
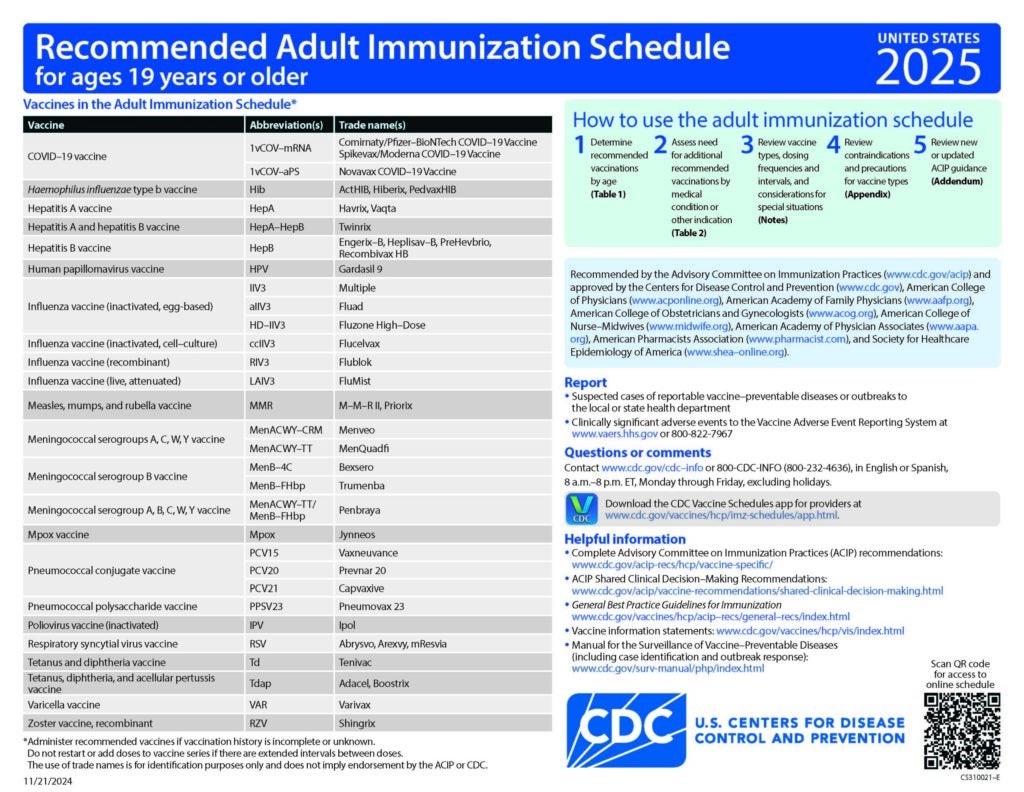
CDC Recommended Schedules
FDA Package Inserts & EUAs
Zoster (shingles): Shingrix Package Insert
GSK