Clinical Resources
Materials for Providers
Alphabetical by Title
Standing Orders for Administering Haemophilus influenzae Type B to Children & Teens
Eligible healthcare professionals may vaccinate children and teens who meet any of the criteria on this form
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Use of Haemophilus influenzae Type b–Containing Vaccines Among American Indian and Alaska Native Infants: Updated Recommendations of the Advisory Committee on Immunization Practices ― United States, 2024
MMWR September 12, 2024 / 73(36);799–802
Licensure of a Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus influenzae Type b Conjugate, and Hepatitis B Vaccine, and Guidance for Use in Infants
MMWR, February 7, 2020, 69 (5);136-139
Prevention and Control of Haemophilus influenzae Type b Disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Recommendations and Reports
MMWR, February 28, 2014; 63(RR01):1-14
Additional Federal Resources
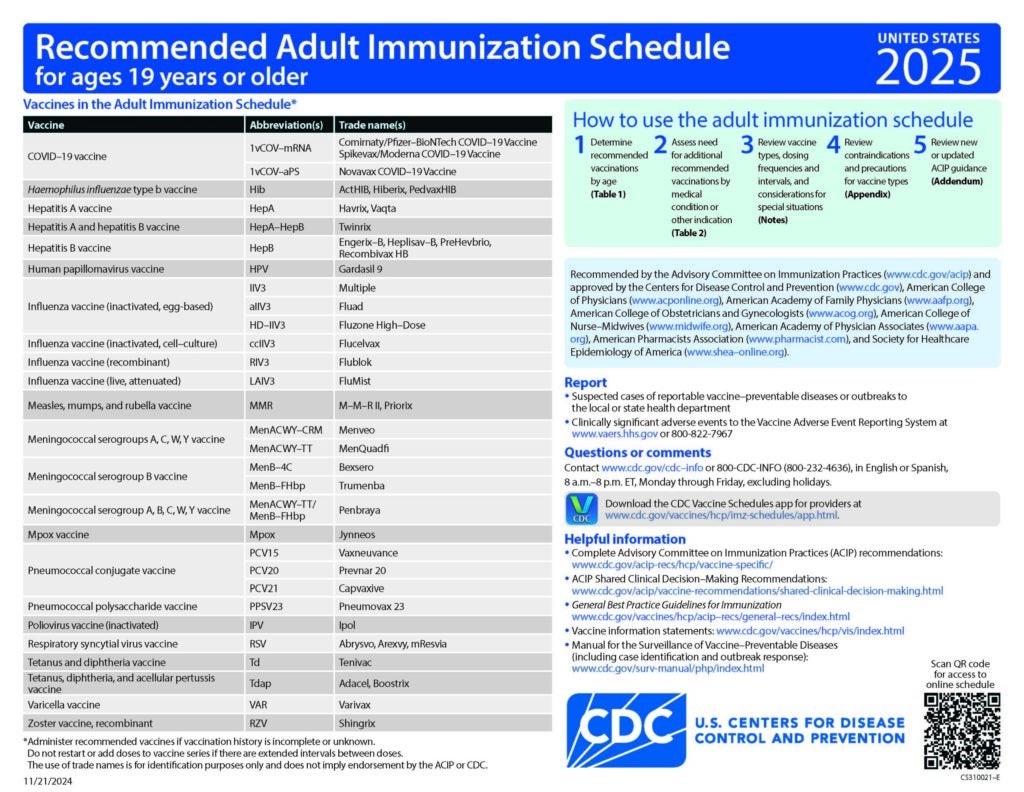
CDC Recommended Schedules
FDA Package Inserts & EUAs
Hib: PedvaxHIB Package Insert
Merck & Co., Inc.
Hib: ActHIB Package Insert
Sanofi U.S.
Hib: Hiberix Package Insert
GSK
Combination Vaccine (DTaP-IPV/Hib): Pentacel Package Insert
Sanofi U.S.
Combination Vaccine (DTaP-IPV-Hib-HepB): Vaxelis Package Insert
MSP Vaccine Company