Clinical Resources
Materials for Providers
Alphabetical by Title
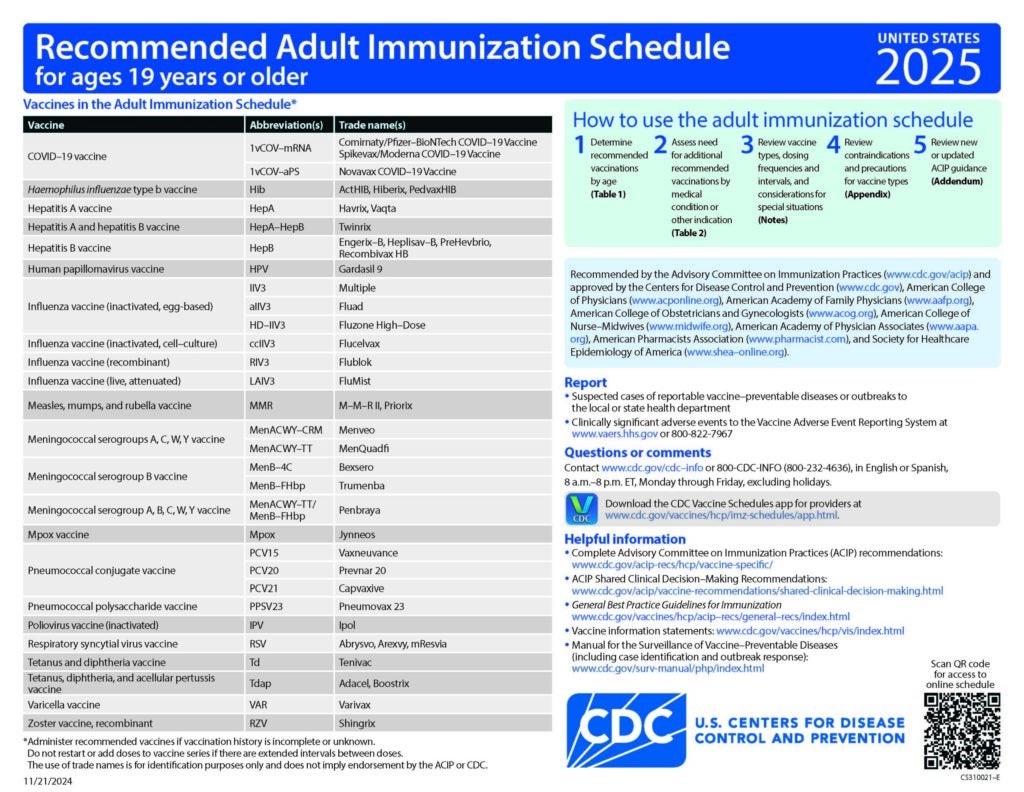
Meningococcal B Vaccine Recommendations by Age and Risk Factor
This piece gives recommendations for MenB vaccine use in people age 10 years or older.
CDC · FDA · State
ACIP Recommendations
Current Recommendations
New Dosing Interval and Schedule for the Bexsero MenB-4C Vaccine: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, October 2024
MMWR December 12, 2024 / 73(49);1124–1128
Use of the Pfizer Pentavalent Meningococcal Vaccine Among Persons Aged ≥10 Years: Recommendations of the Advisory Committee on Immunization Practices ― United States, 2023
MMWR April 18, 2024 / 73(15);345–350
Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020
MMWR, September 25, 2020, Volume 69(9); 1–41
Additional Federal Resources
CDC Recommended Schedules
FDA Package Inserts & EUAs
Meningococcal B: Bexsero Package Insert
GSK
Meningococcal B: Trumenba Package Insert
Pfizer
More from Immunize.org
Unprotected People Stories
Photos & Videos
Immunize.org’s MenB Vaccination Honor Roll
Preventing Outbreaks of Meningitis B in Colleges and Universities
Immunize.org urges colleges and universities to establish policies requiring or recommending meningococcal serogroup B (MenB) vaccination to protect their students and prevent outbreaks. Immunize.org’s MenB Vaccination Honor Roll recognizes champion institutions that have taken the lead in establishing such policies.