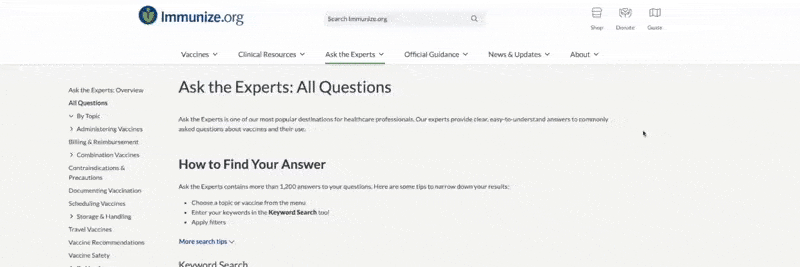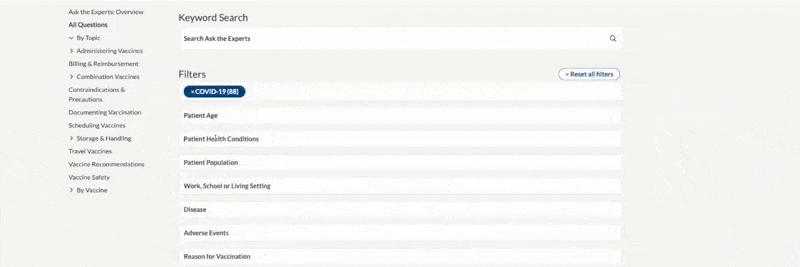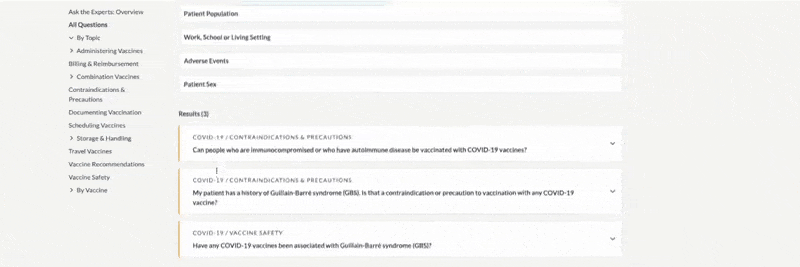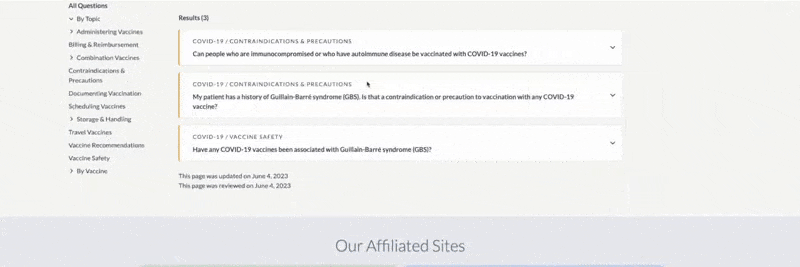
-
HPV (Human Papillomavirus)
Current
Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices
MMWR August 16, 2019; 68(32): 698–702
-
HPV (Human Papillomavirus)
Current
Use of a 2-Dose Schedule for Human Papillomavirus Vaccination: Updated Recommendations of the Advisory Committee on Immunization Practices
MMWR, December 16, 2016; 65(49);1405-8
-
HPV (Human Papillomavirus)
Current
Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices
MMWR, March 27, 2015; 64(11):300-304
-
HPV (Human Papillomavirus)
Current
Human Papillomavirus Vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP)
MMWR, August 29, 2014; 63(RR-5):1-30
-
HPV (Human Papillomavirus)
Archived
Recommendations on the Use of Quadrivalent Human Papillomavirus Vaccine in Males — Advisory Committee on Immunization Practices (ACIP), 2011
MMWR, December 23, 2011; 60(50):1705-8
-
Hepatitis A
-
Hepatitis B
-
HPV (Human Papillomavirus)
Archived
Sexually Transmitted Diseases Treatment Guidelines, 2010
MMWR, December 17, 2010; 59(RR12):1-110
-
HPV (Human Papillomavirus)
Archived
FDA Licensure of Bivalent Human Papillomavirus Vaccine (HPV2, Cervarix) for Use in Females and Updated HPV Vaccination Recommendations from the Advisory Committee on Immunization Practices (ACIP)
MMWR, May 28, 2010; 59(20):626-629
-
HPV (Human Papillomavirus)
Archived
FDA Licensure of Quadrivalent Human Papillomavirus Vaccine (HPV4, Gardasil) for Use in Males and Guidance from the Advisory Committee on Immunization Practices (ACIP) Weekly
MMWR, May 28, 2010; 59(20):630-632
-
HPV (Human Papillomavirus)
Archived
Quadrivalent Human Papillomavirus Vaccine
MMWR, March 23, 2007; 56(RR02):1-24