Clinical Resources
Materials for Providers
Alphabetical by Title
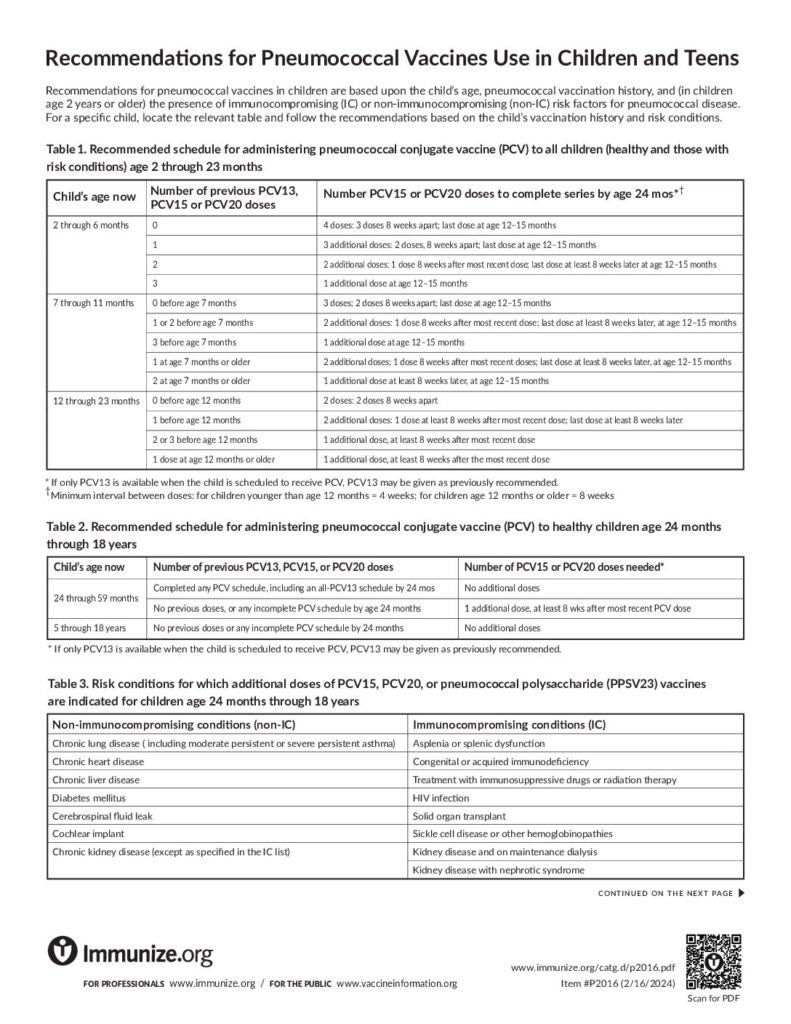
Recommendations for Pneumococcal Vaccines Use in Children and Teens
This piece gives recommendations for pneumococcal vaccines use in children and teens who are healthy or have risk factors.
Standing Orders for Administering Pneumococcal Vaccines to Adults
Eligible healthcare professionals may vaccinate adults who meet any of the criteria on this form
Standing Orders for Administering Pneumococcal Vaccines to Children and Teens
Eligible healthcare professionals may vaccinate children or adolescents who meet any of the criteria on this form. This template is consistent with the American Academy of Pediatrics 2026 Recommended Child and Adolescent Immunization Schedule.
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Additional Federal Resources
- All current and archived ACIP Pneumococcal recommendations
- General Best Practice Guidelines for Immunization
- ACIP Pneumococcal recommendations at CDC
- CDC Pneumococcal Information for Healthcare Professionals
- Summary of Who and When to Vaccinate
- PneumoRecs VaxAdvisor Mobile App for Vaccine Providers
- Pneumococcal Vaccine Timing for Adults
- Pneumococcal Vaccine Recommendations
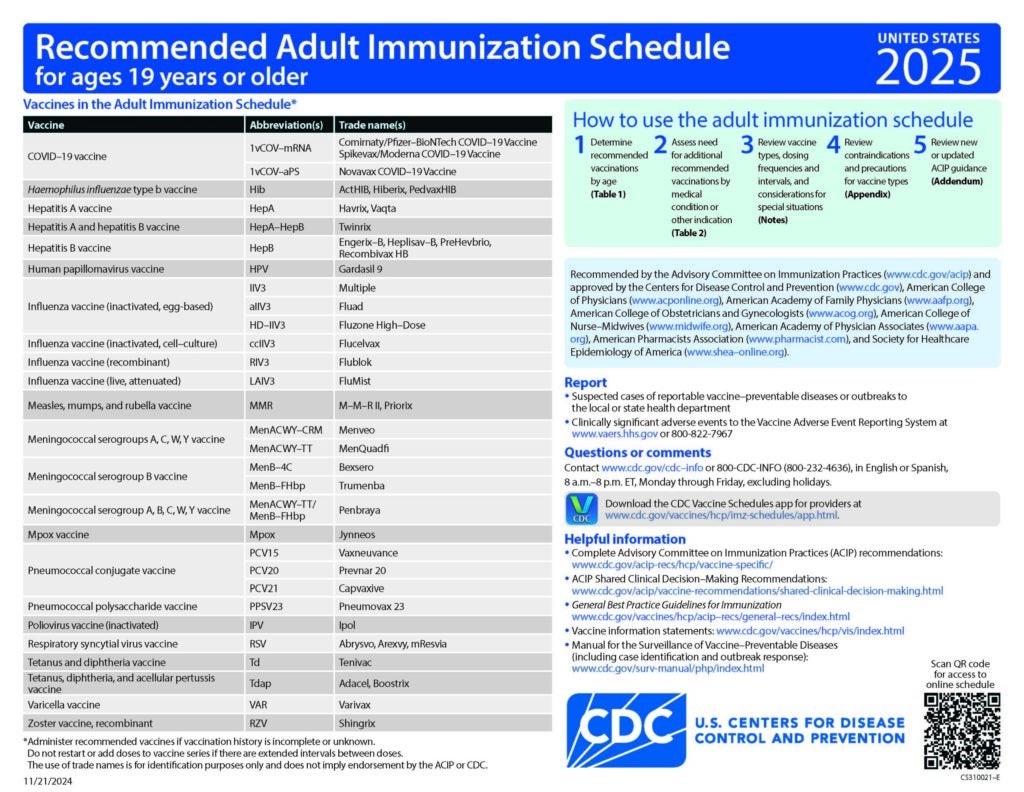
CDC Recommended Schedules
FDA Package Inserts & EUAs
State Policies
Healthcare Professional Organizations
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Travelers’ health information for healthcare providers